 On the Case
On the Case
By Paola Tabaro, MD, and Alex Merkulov, MD
Radiology Today
Vol. 25 No. 6 P. 30
History
A 59-year-old woman with a prior history of fibromyalgia and osteoarthritis presented to her primary care physician due to worsening pain in her left hip and back. The initial imaging workup with radiographs of the left hip and lumbar spine was negative. Given the patient’s worsening pain, an MRI of the left hip and lumbar spine was ordered.
Findings
MRI of the left hip (Figures 1-4) demonstrated an abnormal marrow signal throughout the osseous structures. Marked T2 hyperintense marrow edema of the left femoral head and neck with a subchondral fracture was also noted.
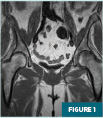
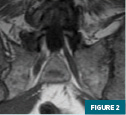
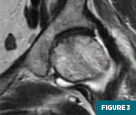
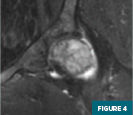
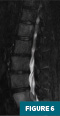
MRI of the lumbar spine (Figures 5 and 6) demonstrated a focal T1 hypointense lytic lesion of the L5 vertebral body with marrow edema and destruction of the posterior endplate with infiltration of the dorsal epidural fat.

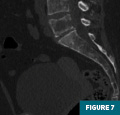
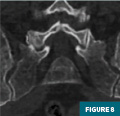
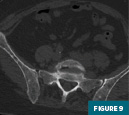
CT of the pelvis (Figures 7-9) demonstrated a lytic lesion of the L5 vertebral body with an associated pathologic compression fracture. Also noted was the heterogeneous appearance of the osseous structures.
Subsequent lab work revealed a monoclonal lambda-free light chain in blood and urine, raising concern for multiple myeloma. An additional workup with a bone marrow biopsy was positive for a plasma cell neoplasm lambda restricted with a high-risk TP53 mutation, 13q and 17p deletion involving the cellular bone marrow, confirming the diagnosis of multiple myeloma.
Shortly after being diagnosed, the patient developed hypercalcemia and was sent to the emergency department for further management. The patient was discharged after the hypercalcemia resolved and initiated chemotherapy as an outpatient. Three weeks later, she presented to the emergency department with abdominal pain and nausea. Inpatient lab work revealed severe pancytopenia and acute kidney injury in the setting of multiple myeloma therapy, and she was treated with plasmapheresis and intensive chemotherapy. During the hospital course, the patient developed C. diff colitis and went into septic shock, expiring shortly after.
Diagnosis
Multiple myeloma
Discussion
Multiple myeloma is also known as plasma cell myeloma and is characterized by neoplastic proliferation of plasma cells that produce a monoclonal immunoglobin known as M protein. The plasma cells undergo proliferation in the bone marrow and can lead to osseous destruction.1
Multiple myeloma most commonly presents in patients aged 40 to 80 years, and the most common clinical manifestations are pain and weakness. Males are more commonly affected, and there is a higher incidence in the Black population. Cases of multiple myeloma can occur sporadically, although most cases are preceded by a premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). Additionally, associations with herbicides, insecticides, benzene, and prior radiation have been cited, and genetic predisposition is also encountered.1
Literature has established a few intermediary stages, which, in addition to MGUS, include plasmacytomas and smoldering multiple myeloma. Smoldering multiple myeloma is an asymptomatic precursor state between MGUS and multiple myeloma, with an average risk of progression to multiple myeloma of 10% per year. Plasmacytoma is defined as a localized neoplasm composed of clonal plasma cells with no evidence of other bone lesions and no evidence of multiple myeloma. Solitary plasmacytoma of bone refers to abnormal plasma cells that form a tumor localized to a specific bone. Extramedullary plasmacytoma is the equivalent to solitary plasmacytoma of bone but presents as a localized mass affecting soft tissue.2
The prognosis for multiple myeloma is variable and ranges from indolent to rapidly progressing symptoms and organ dysfunction. Poor prognostic factors include high-risk cytogenetics (for example, 17p deletion), extramedullary disease, abnormal serum free light chain ratio, and low serum albumin. Treatment involves chemotherapy, peripheral stem cell transplant, and management of complications.2
On a pathophysiologic level, the presence of abnormal plasma cells on bone leads to severe pain and fractures secondary to excessive bone resorption and inhibition of bone formation in the presence of myeloma cells.3
Skeletal imaging plays a critical role in the initial diagnostic evaluation and long-term management of patients with plasma cell disorders.4 Currently available imaging modalities allow the characterization of lytic bone disease, bone marrow infiltration, bone mineral density, and extramedullary involvement.4
Previously, the primary purpose of skeletal imaging in multiple myeloma was the identification of lytic bone disease, pathologic fractures, and evaluation of disease progression. Newer studies have aimed to study the prognostic value of skeletal imaging in the early identification of focal bone marrow involvement and response to therapy.4
Conventional skeletal radiography survey has been the reference standard method for the evaluation of multiple myeloma in the past decade; however, it has very low rates of accuracy since destruction of 30% to 50% of the bone is necessary to detect a lesion.5
Imaging modalities that only depict osteolytic disease may underestimate the extent of the disease when compared with MRI or PET/CT, which can identify focal lesions before significant bone destruction occurs.1 Current guidelines suggest the replacement of conventional skeletal surveys with whole-body cross-sectional modalities due to limited sensitivity. Whole-body cross-sectional imaging has greater sensitivity and can depict marrow involvement.1
Whole-body low-dose (WBLD) CT is a sensitive tool for the detection of the bonedestructive effects in multiple myeloma and the presence of extramedullary disease. CT findings consist of punched-out lytic lesions, expansile lesions with softtissue masses, diffuse osteopenia, fractures, and, rarely, osteosclerosis.5
However, it is important to emphasize that WBLD CT has a good positive predictive value when osteolytic bone lesions are detected, but it has a much weaker negative predictive value. Therefore, if there are no bone lesions at WBLD CT, then a follow-up investigation with whole-body MRI or PET/CT should be performed to exclude lesions and confirm the diagnosis of smoldering multiple myeloma or find lesions that are undetectable with WBLD CT.5
Whole-body MRI is highly sensitive for detecting marrow abnormalities and is currently the most sensitive imaging study for marrow infiltration.1 There are different patterns of marrow infiltration on MRI, such as apparently normal marrow, diffuse or focal marrow involvement, and the salt and pepper variegated type. A low tumor burden is usually associated with the salt and pepper pattern, whereas a high tumor burden usually presents as diffuse or focal involvement.5
Whole-body FDG PET/CT is the modality of choice for identifying extramedullary disease since the focal FDG uptake causes the lesions to be more conspicuous against the soft tissues. The International Myeloma Working Group recommends PET/CT as the initial choice to evaluate extramedullary disease, and it can be used to assess treatment response.1
Upon review of the literature, wholebody MRI is the best imaging modality for the assessment of diffuse marrow involvement. PET/CT is superior to radiography for the detection of bone lesions and can indicate the progression of intermediary stages to multiple myeloma in the presence of focal uptake without osseous destruction.5
— Paola Tabaro, MD, is a radiology resident at UConn Health at the University of Connecticut in Farmington.
— Alex Merkulov, MD, is an associate professor of radiology at UConn Health.
References
1. Wu F, Stephanie Bernard S, Fayad L, et al. Updates and ongoing challenges in imaging of multiple myeloma: AJR expert panel narrative review. AJR Am J Roentgenol. 2021;217(4):775-785.
2. Kyle RA, Durie BG, Rajkumar SV, et al.Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-1127.
3. Angtuaco EJ, Fassas AB, Walker R, Sethi R, Barlogie B. Multiple myeloma: clinical review and diagnostic imaging. Radiology. 2004; 231(1):11-23.
4. Hansford BG, Silbermann R. Advanced imaging of multiple myeloma bone disease. Front Endocrinol (Lausanne). 2018;9:436.
5. Ormond Filho AG, Carneiro BC, Pastore D, et al. Whole-body imaging of multiple myeloma: diagnostic criteria. Radiographics. 2019;39(4):1077-1097.
6. Delorme S, Baur-Melnyk A. Imaging in multiple myeloma. Eur J Radiol. 2009;70(3):401-408.

